Why the lab rats have failed to produce a ‘cure for cancer’
James Fraser explores why, despite our advancing closer and closer to a cure, researchers still rely on animal testing
In the UK, someone dies of cancer every four minutes, accounting for over a third of deaths in under-75-year-olds. Although 50% of people diagnosed with cancer survive for at least ten years, simply mentioning the word is enough to send shivers down the spine, and it’s clear that the proverbial “cure for cancer” remains elusive.
The course of academic progress never runs smoothly, with biomedical research marred in general by publication bias (the binning of inconclusive data in favour of the positive and dramatic), misunderstandings over statistical significance, inadequate peer review (especially by lesser journals), and basic scientific flaws.
Yet cancer poses particular problems to researchers, and these idiosyncrasies account for the failure of many discoveries and novel therapies in the field to live up to their initial hype. Cancer is, in fact, not one but a plethora of diseases – caused, yes, by dysregulation of largely predictable genes, but in patterns that vary widely across people with tumours of the same organ, let alone malignancies of different organs. No two cancers are definitively the same, and thus, no disease is remotely comparable in variability.
This raises important questions about the experimental approaches taken in cancer research. If ostensibly similar cancers are in fact different in vivo, do cancer cells behave in remotely the same way once we grow them in a Petri dish or when grafted into another mammal?
For many years, so-called PDX (patient-derived xenograft) mice have been the bedrock of cancer research. The idea is simple: take tumour cells from a human with cancer, and transplant them beneath the skin of an immunodeficient mouse, itself genetically modified so as not to reject the human tissue. In doing so, we produce a model organism for human cancer (specifically that of the patient in question) to test all manner of putative anti-cancer drugs for humans. Previous studies have proclaimed PDX mice the gold standard of cancer models, providing a more realistic physiological backdrop for malignant cells than culture fluid in a test tube.
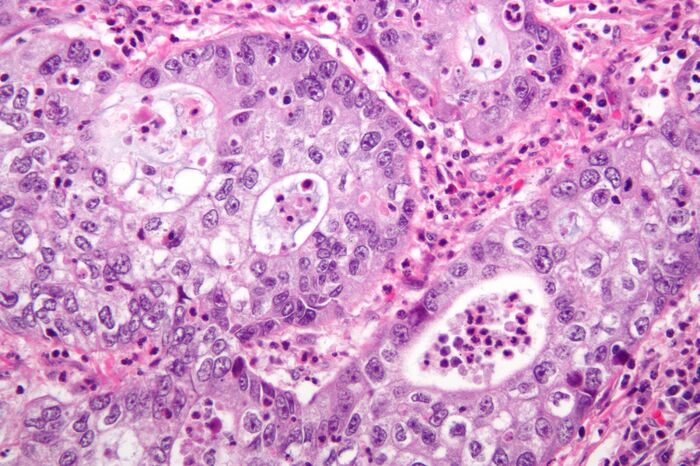
However, analysis from the Broad Institute of MIT and Harvard published in Nature Genetics last November has cast doubt on this pedestalled experimental technique. The researchers tracked copy number alterations (CNAs – duplications or deletions of portions of DNA) across 1,110 PDX samples, covering 24 cancer types. Tumour cells in PDX mice acquired these mutations at the same rates as tumour cells in patients, demonstrating an equivalent level of genomic instability that one would hope for a model to be valuable.
Nevertheless, the genetic alterations themselves were different in nature. Equivalent sections of DNA that had increased in copy number in the xenograft data set were found – in the non-transplanted data set – to have decreased in copy number more often than to have also increased. Equally, sections of DNA that decreased in copy number in the xenograft set had undergone increase in copy number in the majority of cases.
An increase in copy number indicates that a portion of DNA is of benefit to a cancer cell’s survival and proliferation, a decrease one that is a hindrance or at least irrelevant to malignancy; as such, these divergent patterns of change imply that tumours in PDX mice are subjected to physiological selection pressures that do not accurately match up with those within the original tumour environment.
If this is indeed the case, and tumours grafted into mice evolve to differ considerably in genetic and biochemical nature, then testing possible therapeutics on PDX mice is likely to be of limited value. And frankly, why should this necessarily be surprising? Grafting, say, a human pancreatic adenocarcinoma under the skin on the back of a mouse doesn’t strike one as the best simulation of the original tumour environment. What is more, these mice are severely immunocompromised, in spite of years-old research on the relevance of the immune system to cancer development and prevention. To nitpick further, successful xenografting typically necessitates the use of fast-growing cells within a malignant population, introducing a selection bias even before transplantation has occurred.
This may seem purely academic. Indeed, it’s worth saying that the news isn’t all bad: similar contemporary studies have been much less damning, and PDX mice still represent a more effective and flexible experimental paradigm than basic cell culture. But given that fewer than 8% of anti-cancer drugs tested successfully on animal models pass even Phase I clinical trials, there is enormous scope for improved experimental paradigms to accelerate the development of novel therapies.
Already, these are being investigated: transgenic mice with components of the human immune system, reliable ways of seeding and measuring tumours in more appropriate locations, and even the creation of extracellular scaffolds in vitro to mimic the tumour micro-environment more authentically. For now, mouse models are here to stay – but experimental flexibility will be crucial to fast-track therapeutic discovery in a field where cures have proven evasive
 News / SU reluctantly registers controversial women’s soc18 December 2025
News / SU reluctantly registers controversial women’s soc18 December 2025 News / CUP announces funding scheme for under-represented academics19 December 2025
News / CUP announces funding scheme for under-represented academics19 December 2025 Features / Should I stay or should I go? Cambridge students and alumni reflect on how their memories stay with them15 December 2025
Features / Should I stay or should I go? Cambridge students and alumni reflect on how their memories stay with them15 December 2025 Fashion / The art of the formal outfit 18 December 2025
Fashion / The art of the formal outfit 18 December 2025 News / Dons warn PM about Vet School closure16 December 2025
News / Dons warn PM about Vet School closure16 December 2025