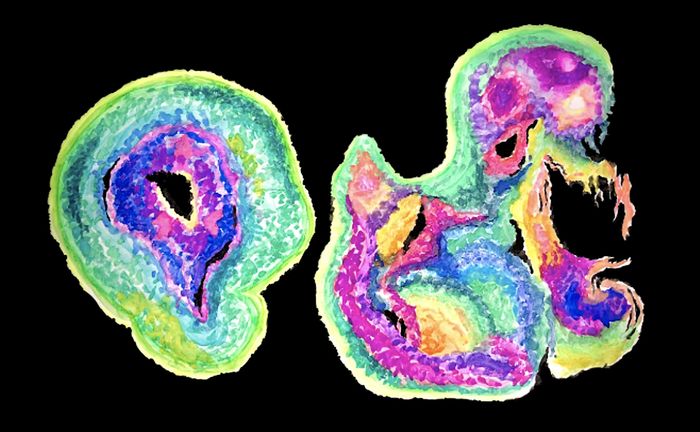
Neanderthal brain organoids throw light on human brain evolution
Research on brain development in archaic humans is often inconclusive. Chloe Li explains how a new study on synthetic brain tissue could change that
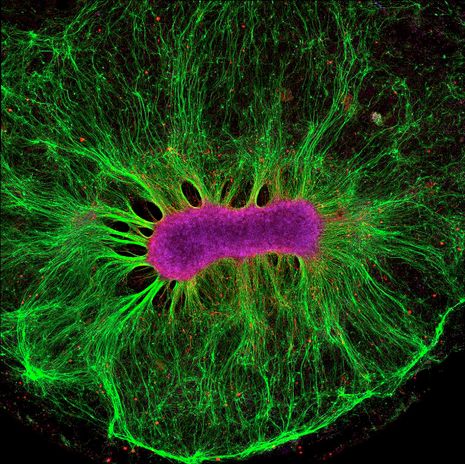
Our closest extinct relatives, the Neanderthals, offer a unique opportunity to understand the genetics underpinning recent evolutionary changes in human brain development. In findings published in February 2021, researchers grew brain-like organoids by inserting a Neanderthal gene variant into human stem cells, bringing us a step closer to this goal.
Since genus Homo debuted on the African plains 3 million years ago, we have emerged as its only survivors, waving goodbye to our closest relatives the Neanderthals and Denisovans some 40,000 years ago. Our tightknit evolutionary history, clarified by high-quality Neanderthal and Denisovan genomes sequenced in 2010 and 2012, have allowed for subtle comparisons that distinguish our species genetically. From genomic analysis, more than 40% of the Neanderthal genome has been consigned to us, leaving behind various phenotypic thumbprints. However, certain regions of the genome contain only human-specific variants. Identifying these conserved sequences and untangling their effect on brain development may uncover unique aspects of our brain evolution. As paleoanthropologist Phillip Gunz puts it, this research could identify “the most recent innovation in our brain”.
“The uncertain and indirect nature of computer reconstruction in studying development often brings about inferential stalemates”
Through genome-wide comparisons, Alysson Moutri’s team at the University of California, San Diego found 61 genes that have a fixed human variant unshared by Neanderthals and Denisovans. Of these, NOVA1 was identified as a key gene for nervous system development. It does so by regulating alternative splicing, which allows one gene to produce multiple types of proteins. Unsurprisingly, NOVA1 mutations are associated with brain disorders. The human NOVA1 variant differs in a single base from the variant in Neanderthals and Denisovans and likely altered our brain development in a crucially advantageous way. The question is of course, what and how.
There is no easy answer when it comes to the thorny subject of brain development. Soft brain tissues are not preserved in the fossil record, so research has conventionally relied on fossilized skulls. As the brain is tightly encased, the impression it makes on the inner surface of the cranium (braincase) can be visualized by an endocast, then computationally analysed to estimate brain size and the shape of the outer cortex. Broca’s and Wernicke’s area, brain regions associated with speech production and comprehension, have been studied in this way to infer linguistic abilities in our archaic relatives. However, neither brain structure nor its developmental process can be directly revealed by this.
The uncertain and indirect nature of computer reconstruction in studying development often brings about inferential stalemates. Researchers Gunz (2018) and Ponce de Leόn (2016) tried to infer the timing of anatomical divergence by comparing human and Neanderthal braincases from birth to adulthood. While Gunz showed that braincases of Neanderthal and human newborns are structurally similar, Ponce de Leόn criticised their method of reconstruction for overrepresenting similarities and concluded the opposite. Hence, whilst Gunz identified postnatal formation of neuronal connections as the driver of divergence, Ponce de Leόn clashingly emphasized the importance of prenatal growth.
“The human NOVA1 likely evolved alongside a suite of genetic changes, the culmination of which creates the morphology we see today”
The breakthrough from Moutri builds on recent development of brain organoids as a new approach to studying brain development. These are brain-like tissues developed from human stem cells and grown on petri dishes. They allow researchers to directly recreate developmental processes from time zero in vitro, and test the effect of human-specific gene variants with greater accuracy and control than was previously possible with animal models. Madeline Lancaster, a pioneer of this technique, most recently used it to compare early neurogenesis between humans and apes. In her paper published on March 24th, she identified a new transition state in neuron development that differs in timing and morphology between humans and apes, alongside one of its key protein regulators, which potentially explain our large brain size. With the same approach, Moutri replaced the human NOVA1 variant with the archaic variant inside human pluripotent stem cells using CRISPR-Cas9 technology. They then cultured the cells into organoids and compared them.
The most striking difference was in morphology: organoids carrying the human variant were smooth and spherical whereas ones with the archaic variant were smaller and roughly textured. Moutri investigated the molecular underpinnings of these changes and found that cells with the archaic variant divide more slowly and experience cell death differently, which led to an aberrantly structured cell layer. This change was reflected in gene expression differences: 277 genes were expressed differently, most of which are involved in cell proliferation, neuronal connectivity and synaptic development.
As researchers followed these genetic changes downstream, they saw that levels of synaptic proteins differed significantly in the presence of the archaic mutation. This caused neurons to fire out of sync, which would likely alter neuronal connections and long-term development. As predicted, growing brain organoids diverged in cell composition after 2 months: the archaic organoids contained different proportions of neurons, glia and progenitor cells from human organoids.
Whilst insightful, Moutri stressed that their results do not represent true Neanderthal brains nor conclude the cause of anatomical differences. As human stem cells provide a genetic background different to what the archaic variant historically coexisted with, its insertion may have sparked different interactions and generated a completely novel phenotype that is neither Neanderthal nor Denisovan. The human NOVA1 likely evolved alongside a suite of genetic changes, the culmination of which creates the morphology we see today. Further experiments with a wider diversity of stem cell lines may better clarify the layered effects of NOVA1.
Nevertheless, engineered organoids provide a promising platform to investigate the function of human-specific gene variants in brain organization and development. Some researchers are already looking at developing organoids that receive signals and respond to environmental changes. While organoids cannot compare to fossils, which are true phenotypic products of development, together they may improve understanding of the evolution of the human brain.
 News / Uni members slam ‘totalitarian’ recommendation to stop vet course 15 January 2026
News / Uni members slam ‘totalitarian’ recommendation to stop vet course 15 January 2026 Comment / Will the town and gown divide ever truly be resolved?12 January 2026
Comment / Will the town and gown divide ever truly be resolved?12 January 2026 Science / Why smart students keep failing to quit smoking15 January 2026
Science / Why smart students keep failing to quit smoking15 January 2026 Features / How sweet is the en-suite deal?13 January 2026
Features / How sweet is the en-suite deal?13 January 2026 Arts / Fact-checking R.F. Kuang’s Katabasis13 January 2026
Arts / Fact-checking R.F. Kuang’s Katabasis13 January 2026