Making sense of the English COVID variant
Laura Ryan explains the origins of the new COVID variant and how it may shape the pandemic
Whilst wading through the tsunami of articles published in the wake of the most recent wave of COVID restrictions, I noticed two strikingly different attitudes towards the new SARS-CoV-2 lineage first identified in Kent, termed B.1.1.7. The first; that the discovery of this variant could change the course of the pandemic, and must radically alter our government’s strategy going forward. The second; that viruses mutate all the time, that mutation is natural, and that it’s usually nothing to worry about. So which is true? Well, the answer is both.
Mutations do indeed occur naturally – they are how organisms evolve. When viruses replicate in human cells, they make copies of their genome. The replication process is not perfect, and mistakes are inevitably made, leading to changes in the genetic code (mutations). Different viruses have different rates of error when copying their genomes, so they mutate at different speeds. SARS-CoV-2 is able to proofread the code of the new genetic material it synthesises, so it has a relatively slow mutation rate – about half of that of influenza, and a quarter of that of HIV. But it still makes mistakes; typically strains accumulate about two errors per month.
The genetic code can be thought of as a set of instructions for making proteins. Most mutations have no effect on the virus, because not every letter of the code is equally important, and some can be mistakenly swapped without changing the instructions necessary to build proteins correctly. These mutations are referred to as “silent”, or “synonymous”. They have no effect on the virus’s transmission, or ability to cause disease. However, occasionally a mutation occurs in a position that does modify the protein encoded by the gene. These are called “non-synonymous” mutations, and they can change the way the virus behaves, making it either more or less able to compete with other strains in its environment.
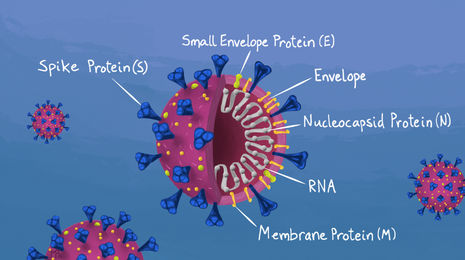
Interestingly, the B.1.1.7 lineage carries more mutations than researchers would expect for a virus with the mutation rate of SARS-CoV-2, particularly in the region that encodes for the viral spike protein, which allows the virus to enter cells. The vast majority of new lineages of SARS-CoV-2 identified around the globe carry only a couple of distinct mutations. In contrast, the new strain identified in Kent has accumulated around 20 different lineage-specific mutations. Additionally, a higher proportion of these mutations are non-synonymous than is typical, indicating that this lineage has evolved in response to some selective environmental pressure.
How can this be explained?
One current theory relates to the treatment of chronically ill COVID patients with convalescent plasma – plasma from the blood of recovered patients. Convalescent plasma is packed full of antibodies against the viral strain(s) that infected the donor, intended to neutralise disease in the recipient. The catch is that chronically infected or immunodeficient COVID patients with high viral loads often harbour an unusually diverse viral population, with an increased number of accumulated mutations - a large proportion of which are non-synonymous. If these patients are treated with convalescent plasma, the antibodies present are most effective at suppressing viral strains encountered by the donor. This may unintentionally enable novel strains carried by the patient to thrive, and potentially be transmitted. Such a situation could prove to be significant, as some scientists have cited the lack of selective pressure on a population scale as evidence that we shouldn’t be too concerned about dangerous variants arising. As the virus is successfully spreading between individuals, there is currently very little pressure on the virus to evolve on the level of the population (although this may change with widespread vaccination). But perhaps it is pressures within, rather than between, individuals that we should be watching closely.
How different is the new strain, and what does this mean for vaccines?
From analysis of the specific mutations in the B.1.1.7 lineage, in concert with experimental data and animal models, researchers initially predicted an increase in transmissibility. Notably, a mutation in the receptor binding domain (N501Y) was known to strengthen the interaction between the viral spike protein and the human ACE2 receptor, helping the virus to latch onto and enter cells. Another mutation (P681H) has been shown to promote infection of respiratory epithelial cells. Confirmation came this week in a report from Imperial College that used epidemiological data to demonstrate that the B.1.1.7 lineage is as much as 50% more transmissible than previous strains (an increase in R of between 0.4 and 0.7). The report also indicated that the average age of patients testing positive for the new variant is significantly lower than that of previous strains. As of yet there is no evidence that the variant causes more severe disease, but this can’t be ruled out.
"We shouldn’t be complacent; that this particular strain has accumulated such a constellation of different mutations is cause for concern."
What does this mean for our vaccines? The vaccines are designed to trigger the production of antibodies that recognise the spike protein which coats the outside of SARS-CoV-2 viral particles. It is possible for specific viral mutations to significantly alter the spike protein, which could render the virus resistant to antibody recognition. We shouldn’t be complacent; that this particular strain has accumulated such a constellation of different mutations is cause for concern. We can’t be certain that immunity from vaccination or previous infection will still be as robust as we would hope. But at the moment, it is a question of the degree of protection – it is highly unlikely that the changes we have so far seen in the spike protein of the B.1.1.7 linage are enough to evade our vaccines entirely. The new South African variant (501.V2) poses greater cause for concern, as it carries additional mutations in crucial regions of the spike protein that could significantly affect vaccine efficiency. But we shouldn’t panic - multiple vaccines are available, more are in development, and all can be altered as the virus evolves, so there is good reason for cautious optimism.
 Comment / Plastic pubs: the problem with Cambridge alehouses 5 January 2026
Comment / Plastic pubs: the problem with Cambridge alehouses 5 January 2026 News / Cambridge academics stand out in King’s 2026 Honours List2 January 2026
News / Cambridge academics stand out in King’s 2026 Honours List2 January 2026 News / Cambridge businesses concerned infrastructure delays will hurt growth5 January 2026
News / Cambridge businesses concerned infrastructure delays will hurt growth5 January 2026 News / AstraZeneca sues for £32 million over faulty construction at Cambridge Campus31 December 2025
News / AstraZeneca sues for £32 million over faulty construction at Cambridge Campus31 December 2025 Interviews / You don’t need to peak at Cambridge, says Robin Harding31 December 2025
Interviews / You don’t need to peak at Cambridge, says Robin Harding31 December 2025