Cambridge Spotlight: OLEDs and Polymer Electroluminescence
Raghav Chandra introduces the Cavendish Laboratory’s research on organic light-emitting diodes (OLEDs) and also discusses the future of OLEDs.

You see OLED displays in smartphones, computer monitors and even TV screens; but did you know that some of the first practical OLEDs were made in the Cavendish Laboratory at the University of Cambridge?
“Thirty years ago, Jeremy Burroughes’ PhD at the Cavendish Laboratory culminated in the discovery that certain polymers were capable of emitting visible light at high efficiencies when an electric current was passed through them by a phenomenon called electroluminescence.”
Organic light-emitting diodes, or OLEDs, are dominating the markets for displays due to their ‘unsurpassed display, brightness, color and infinite contrast.’ They are predicted to be used in over 50% of smartphones sold worldwide by 2023, replacing lower quality, less efficient LCDs.
Thirty years ago, Jeremy Burroughes’ PhD at the Cavendish Laboratory culminated in the discovery that certain polymers were capable of emitting visible light at high efficiencies when an electric current was passed through them by a phenomenon called electroluminescence. Among these were Poly (p-phenylene vinylene) (PPV) which emitted yellow-green light.
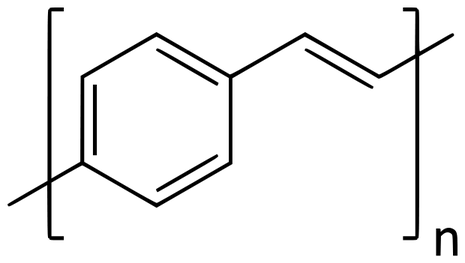
This process can be explained by quantum mechanics. The polymers used were conjugated, meaning that some of their electrons were spread across the whole polymer chains in π molecular orbitals, like how electrons are delocalised in benzene. This also makes them somewhat conductive. A molecular orbital is just a region in a molecule which can be occupied by up to two electrons and which has a specific energy. Molecular orbitals are normally filled from the lowest energy to the highest energy, and π just refers to a specific type of them. Of these, two of particular importance are the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO).
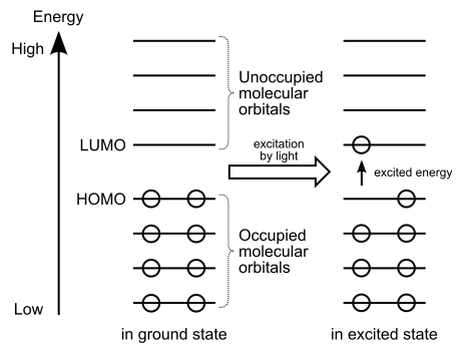
If electrons are removed from the molecule, they will typically be removed from the HOMO and if they are added, they will enter the LUMO. If a current is applied, an electron can be removed from the HOMO and another electron added to the LUMO. The electron added to the LUMO moves to the new ‘hole’ in the HOMO and emits a photon with the energy difference of the HOMO and LUMO (the band gap); this is similar to how sodium-vapour streetlamps work in which a current is passed through, electrons move to higher orbitals and emit light as they return to their original orbitals. As conjugation increases, the molecular orbitals become closer in energy and the band gap decreases. If this band gap energy corresponds to a photon in the visible spectrum, then we see light emitted.
However, impurities and kinks in the polymers can stop this process by introducing “traps,” with an energy between the HOMO and LUMO. Instead of the electron and hole directly recombining to emit light, they can recombine via the trap to release two phonons (vibrations which usually cause heating). Previously, there was little interest in polymer luminescence as in polyacetylene, the most widely studied of these materials, this was very inefficient due to these traps. Burroughes discovered that PPV is much more efficient as it is a more rigid polymer less prone to kinks and can be made into high-purity films. By sandwiching a 100 nm film of this between thin layers of aluminium and indium oxide (a transparent conductor), a large-area display could be made. The resulting screen was robust and stable as a result of using polymers rather than small molecules which were prone to structural changes.
Older liquid-crystal displays (LCDs) use a backlight with two perpendicular polarisers (polarisers only allow a certain orientation of light to pass through) and a “nematic” between them which rotates light 90° so without an applied voltage, light passes through the screen. When a voltage is applied, the molecules change orientation and stop rotating light, stopping the light from passing through. A small amount of light however still passes through and so pixels never appear truly black.
With OLEDs, each molecule acts as an individual LED; there is no backlight, allowing for true blacks and making them thinner, lighter and more efficient. They can also be made flexible, allowing for foldable and rollable displays, and even transparent, for heads-up displays and augmented reality.
“Their use in lighting is being investigated as they do not rely on rare and expensive elements that normal LEDs require such as gallium and arsenic. The future of OLEDs, as we see it, is bright.”
Burroughes, alongside other researchers and co-authors, (including now Cavendish Professor of Physics Richard Friend), started a company, Cambridge Display Technologies (CDT), to commercialise the discovery. This became the first University of Cambridge spin-out to go public when it was listed on the NASDAQ stock exchange in 2004 and it was subsequently acquired by Sumitomo Chemical in 2007 for $285 million.
Now OLED displays are used in Apple iPhones, LG TVs, Sony Computer Monitors, Playstation VR headsets, Samsung foldable smartphones and many more devices. Development is still ongoing to improve flexibility, efficiency, affordability and stability. Foldable devices such as the Samsung Fold are gradually becoming more durable and mainstream. They could be used in transparent displays integrated into windscreens or built into goggles for augmented reality. Their use in lighting is being investigated as they do not rely on rare and expensive elements that normal LEDs require such as gallium and arsenic. The future of OLEDs, as we see it, is bright.
 News / CUP announces funding scheme for under-represented academics19 December 2025
News / CUP announces funding scheme for under-represented academics19 December 2025 News / SU reluctantly registers controversial women’s soc18 December 2025
News / SU reluctantly registers controversial women’s soc18 December 2025 News / Cambridge welcomes UK rejoining the Erasmus scheme20 December 2025
News / Cambridge welcomes UK rejoining the Erasmus scheme20 December 2025 Features / Should I stay or should I go? Cambridge students and alumni reflect on how their memories stay with them15 December 2025
Features / Should I stay or should I go? Cambridge students and alumni reflect on how their memories stay with them15 December 2025 Film & TV / Timothée Chalamet and the era-fication of film marketing21 December 2025
Film & TV / Timothée Chalamet and the era-fication of film marketing21 December 2025