An Introduction to Vaccine Challenges: Part 3
Dr Hicham Bouabe writes this week on the different methods of vaccine administration and what effect this has on efficacy of vaccines.

The efficacy and safety of vaccines depend on the type and immunogenicity of the antigen, the type of the adjuvant, the immunological background of the vaccine recipients, as well depend on the administration route of the vaccine.
We went in the previous part of this series through the advantages and disadvantages of the different types of vaccines. In the following we will discuss the impact of the delivery route on the efficacy and safety of a vaccine.
Not all roads lead to Rome
The route of vaccination has an impact not only on the safety of vaccines, but also on the location, type and magnitude of immune responses.
The main routes used to administer vaccines are mucosal, including oral and intranasal administration; or parenteral, including subcutaneous (SC), intradermal (ID) and intramuscular (IM) administration.
Advantages and disadvantages of ID route of vaccination
Although intramuscular (IM) vaccines usually generate weaker immune responses compared to intradermal (ID) and oral immunization, most vaccines are administered via IM needle injection because of their higher safety. A major challenge of ID vaccination is the correct placement of the needle to enable accurate ID delivery.
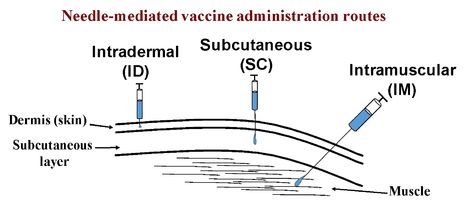
However, IM vaccination often requires multiple doses with an interval between several weeks to few months, in order to elicit an adequate immune response. In addition, the administration of vaccines by injection is cost-ineffective, as it requires the production of injection devices, safe disposal of the used needles and syringes, and employment of trained medical staff to perform the injections. Moreover, IM vaccination usually doesn’t induce mucosal immunity, a ‘strategic’ immune defence of the body against invading pathogens.
Advantages and disadvantages of mucosal routes of vaccination
Respiratory, gastrointestinal and genital mucosal surfaces are used by many pathogens as primary entry points. For instance, poliovirus, rotavirus, norovirus, adenovirus, Vibrio cholerae, Helicobacter pylori, and Salmonella invade orally and infect primarily the gastrointestinal tract. Coronavirus (e.g. SARS, COVID-19), rhinovirus, influenza virus, and Mycobacterium tuberculosis enter into the body via the respiratory tract (through the nose/mouth), infecting first the lining of the lung. The genital tract is the major entry site of pathogens such as Syphilis, Chlamydia, Neisseria gonorrhoeae, human papillomavirus (HPV) and HIV.
“Optimally, the vaccine delivery route should mimic the natural route of infection.”
The protection is supposed to be most effective when the vaccine provokes immune responses also at the initial site of pathogen invasion and transmission, namely at mucosal surfaces, the main entry site for many pathogens. Optimally, the vaccine delivery route should mimic the natural route of infection.
Mucosal immunity can be induced by oral and intranasal vaccines. Mucosal vaccination has several advantages above parenterally delivered vaccines (SC, ID, IM). While the latter induce mainly systemic immune response (circulating in the blood) consisting mainly of serum immunoglobulin G antibodies, mucosal vaccines can lead simultaneously to systemic (e.g. serum IgG) and local mucosal immune responses. A hallmark of mucosal immune response includes the secretory antibody (immunoglobulin) isotype A, secretory IgA (sIgA), a protease-resistant antibody. IgA has several functions in mucosal defence including the entrapment of pathogens preventing them from attachment to the epithelium and invasion of the mucosa into surrounding tissues and organs.
In addition, in some cases mucosal vaccines induce rapid immune responses compared to parenteral vaccination, which makes them more suitable in halting the rapid spread of endemic infections. Mucosal immunization has also been reported to induce cross-reactive antibodies. Cross-reactive antibodies provide broader protection, as they have the ability to recognize more than one antigen, which increases the ability of the recognition of and protection against closely related and/or mutated pathogens.
Furthermore, mucosal vaccines do not need to be injected, and thus don’t require trained medical staff for administration, and consequently are more suitable for mass vaccination.
“Oral vaccination is inappropriate against pathogens that infect the respiratory tract such as coronavirus”
Currently, there are only two approved intranasal vaccines, both contain live attenuated influenza viruses. FluMist was approved in 2003 in the United States, and Nasovac H1N1 vaccine was approved in India in 2010.
Licensed oral vaccines using live attenuated pathogens include: Vivotif (Crucell) vaccine against Salmonella enterica serovar typhi (S. typhi) that causes typhoid fever; Sabin vaccine, an oral polio vaccine (OPV); RotaTeq (Merck) and Rotarix (GlaxoSmithKline) vaccines against rotavirus that causes severe diarrhoea; And Dukoral (Crucell), a vaccine against V. cholera.
However, oral vaccination is most suitable for enteropathogens (pathogens that infect the gastrointestinal tract), such as poliovirus and rotavirus, but inappropriate against pathogens that infect for example the respiratory tract (e.g. the lungs), such as coronavirus and influenza virus.
Furthermore, mucosal surfaces are challenging for vaccine delivery due to physiological barriers, including pH and proteolytic enzymes, which might be harmful for some orally or intranasal administered vaccines, especially subunit vaccines and inactivated vaccines, resulting in their destruction before they initiate any immune response. To overcome this limitation, vaccines need to be encapsulated in appropriate substances that protect them from the harsh physiological environment.
Moreover, as discussed previously, live oral vaccines might show serious adverse effects, including reversion of the attenuated pathogen to virulence via mutations, or induction of harmful inflammation.
Reconsideration of intravenous route of vaccination
Intravenous injection is normally not used for human vaccination, as it usually induces relatively low immune response compared to other administration routes, and more importantly, since it is administered systemically (through blood), it can cause anaphylaxis (serious allergic reaction).
However, a recent study on rhesus macaques, a monkey that closely mirrors human tuberculosis (TB) infection, has shown that TB immunisation (using BCG vaccine) given intravenously conferred almost complete protection from pulmonary TB, compared to intradermal or aerosol vaccine delivery which conferred no or modest protection from pulmonary TB (Darrah et al. Nature, 2020).
Tuberculosis is a disease caused by Mycobacterium tuberculosis, which may infect the lungs causing pulmonary TB (a deadly disease), or infects other parts of the body (outside the lungs) causing several forms of extrapulmonary TB.
The only currently licensed vaccine against TB in humans is a live attenuated bacteria strain, known as bacille Calmette–Guérin (BCG), which is related to Mycobacterium tuberculosis. BCG is usually administered intradermally and provides protection mainly against some forms of extrapulmonary TB in infants, but isn’t as efficient in preventing the transmissible pulmonary TB, which is the dominant disease form in adults.
Whether intravenous delivery of BCG would also confer optimal protection against pulmonary TB in humans needs still to be elucidated. Nonetheless, this study clearly emphasises the impact of the administration route on vaccine efficacy.
In the next part of this series, we will go through the challenges imposed by viruses, the immune system, and experimental procedures.
 News / Judge Business School advisor resigns over Epstein and Andrew links18 February 2026
News / Judge Business School advisor resigns over Epstein and Andrew links18 February 2026 News / Gov grants £36m to Cambridge supercomputer17 February 2026
News / Gov grants £36m to Cambridge supercomputer17 February 2026 News / CUCA members attend Reform rally in London20 February 2026
News / CUCA members attend Reform rally in London20 February 2026 News / Union speakers condemn ‘hateful’ Katie Hopkins speech14 February 2026
News / Union speakers condemn ‘hateful’ Katie Hopkins speech14 February 2026 News / Hundreds of Cambridge academics demand vote on fate of vet course20 February 2026
News / Hundreds of Cambridge academics demand vote on fate of vet course20 February 2026