An Introduction to Vaccine Challenges: Part 2
Dr. Hicham Bouabe explores the science behind vaccines in particular relation to the coronavirus pandemic in the second instalment of this 5-part article series.

In the first part of this series, I emphasized the vital role of vaccination in preventing the outbreak of infectious diseases, and summarized briefly the major challenges in vaccine development. In the present second part we will go in details through different types of vaccines and adjuvants, of which advantages and disadvantages need to be carefully considered when designing a vaccine against a given pathogen.
Vaccine types
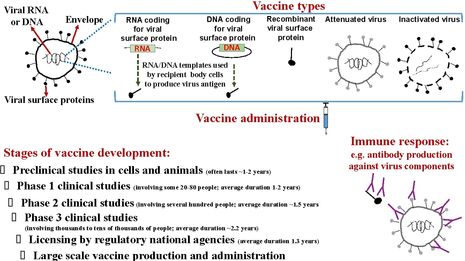
There are different types of vaccines. Each type has/requires a different formulation, which determines how they can be stored and administered, how effective and safe they are, for whom they can be used, and what adverse events they harbour. The advantages and disadvantages of each vaccine type need to be balanced very carefully when designing a vaccine against an infectious disease.
Live attenuated vaccines
One of the earliest vaccines in modern history, smallpox vaccine, is composed of live (attenuated) vaccinia virus (belongs to the family of Poxviruses which also includes the variola virus that causes the smallpox infectious disease in humans). The vaccinia virus is relatively harmless to humans, but because of its similarity to the threatening variola virus, it induces protective immunity also against the latter.
“Live attenuated vaccines are often not suitable... for individuals with compromised immune systems”
Today this type of vaccination, by using live attenuated microorganisms, is routinely used against several life-threatening viruses including measles, mumps, rubella and polio viruses.
Live, attenuated (weakened) vaccines are the most potent vaccines, because they reproduce a normal infection, but with limited infectiveness and thus without causing a disease (or they cause a very mild disease). Therefore, they induce a significantly high and broad spectrum of immunogenicity (induction of cellular and mucosal immune responses, including cross-reactive antibodies), of which immunological memory lasts a long time. A single vaccine administration is often sufficient to induce long-term, sometimes lifelong, protection against the target pathogen.
However, live attenuated vaccines are often not suitable for all people. For example, they aren’t applicable for individuals with compromised immune systems, such as the elderly, HIV/AIDS patients, people undergoing chemotherapy, and people experiencing diseases that weaken the immune system. Those with compromised immune systems are most vulnerable in pandemics.
Furthermore, the most important limitation of live vaccines is their adverse events, as they have the risk of inducing inflammatory or autoimmune diseases, or the risk of reversion to virulence via mutations, and thus inducing the disease they are meant to protect against.
For example, although rare, cases of vaccine-associated paralytic polio (VAPP) have occurred. Oral polio vaccine (OPV) is usually used to prevent poliomyelitis, an infectious neurological disease caused by the poliovirus that can infect the spinal cord and brain, resulting in muscle weakness and inability to move (paralysis). VAPP is caused when the administered attenuated polio vaccine strain gets mutated in the intestine and reverts to virulence - normal damaging viral activity. VAPP can occur in the recipients of the vaccine or in unvaccinated people who come in contact with the stool or oral secretions of the vaccine recipients. Therefore, the World Health Organization (WHO) has recommend since 2016 that a child should receive at least one dose of the inactivated polio vaccine (IPV) (containing killed polio viruses), before or along with the first administration of the OPV (with live attenuated polio viruses). In this way, before any possible reversion of the OPV occurs (which can take months), the IPV will induce a certain level of protective immunity against a given emergent reverted virulent polio virus, and thus limits the incidence of paralysis from VAPP.
Moreover, the first registered vaccine against rotavirus, Rotashield (Biovirx), was approved in the US in 1998. The enteropathogenic rotavirus causes vomiting and severe diarrhoea that can lead to serious dehydration (loss of body fluid). It affects mainly babies and young children. The dehydration can lead to death if not treated. The vaccine consisted of four live attenuated strains, which were administered orally. Although the vaccine successfully passed initial clinical studies, it was withdrawn 9 months after its approval. The vaccine induced a number of cases of intussusception, compared with non-vaccinated children. Intussusception is an abnormal condition in which a part of the gut folds into the section immediately ahead of it (prolapse), causing obstruction.
Inactivated vaccines
Whole, killed (inactivated) vaccines that are produced by killing the pathogen with heat, chemicals, or radiation, have been applied as alternatives to live vaccination. Inactivated vaccines are routinely used for example against hepatitis A, flu (influenza virus), polio and rabies.
Inactivated vaccines are more safe than live vaccines because they don’t induce a strong inflammation and, since they are dead, there is no risk of reversion-related disease enhancement, as the case with live attenuated vaccines. Furthermore, because they are dead and induce relatively mild inflammation, inactivated vaccines are more suitable than live vaccines for people with compromised immune systems.
However, not every disease-causing microorganism can be effectively targeted with an inactivated vaccine. One of the big disadvantages of inactivated vaccines is the induction of weak immune responses, and often the failure to induce long-lasting immunity. Therefore, inactive vaccines often need the help of adjuvants (which cause inflammation and boost the immune response of a vaccine), and sequential multiple administrations of the vaccines are required to reach an optimal level of protective immunity against the target pathogen.
“Adjuvants can have not only profound effects on the type, quality and magnitude of the immune response to a given antigen, but also different adverse effects.”
In addition, in contrast to live vaccines, inactivated vaccines usually don’t induce effective mucosal immunity, a very important component of the immune system against several pathogens, especially those that enter into the human body via the mucosal membranes of the respiratory, digestive, or genital tracts. Mucosa is a membrane that lines various cavities in the body and covers the surface of internal organs. The mucosa is composed of a layer of epithelial cells which establishes a physical barrier against invaders such as pathogens, and is populated with cells of the immune system, building the so-called mucosal immune system. The mucosal immunity is very important in preventing infections of the throat, lungs, gastrointestinal and urogenital tracts.
Mucosal immunity can be induced via inclusion of appropriate adjuvants into the inactivated vaccines, and by designing a route for their administration that favours mucosal immune response (discussed in the next part of this series).
Subunit vaccines
A smart, thorough, controlled and measurable alternative vaccination method to overcome the drawbacks of the whole vaccines is the use of particular fragments of the pathogen (called antigens), typically its surface protein(s), sugar molecules (called polysaccharides), fragments of its surface proteins (called peptides) or its deactivated poisonous proteins (called toxins). Examples of this so-called ‘subunit vaccines’ that are routinely used are hepatitis B vaccine, diphtheria vaccine, tetanus vaccine and pertussis (whooping cough) vaccine.
The antigens used for subunit vaccination can be prepared in the laboratory, purified and administered into people; or, instead, a genetic sequence (DNA or RNA) that encodes the information for forming the amino acid sequence of the antigen, can be administered. In this case, the cells of the vaccine recipient produce the antigen using the DNA/RNA vaccine template. There are many different delivery platforms for antigen-coding genetic sequences especially for DNA, which I’m not going to go into here. However, it should be mentioned that subunit vaccines usually need adjuvants to boost their immunogenicity.
The big challenge of subunit vaccines is to find an appropriate subunit (antigen) of a pathogen which shows optimal immunogenicity and which, along with a suitable adjuvant, produces not only an effective primary immune response against the target pathogen but, more importantly, a lasting protective immunological memory.
The challenge to determine a suitable adjuvant for a given vaccine
Adjuvants can have not only profound effects on the type, quality and magnitude of the immune response to a given antigen, but also different adverse effects. Adjuvants may overshoot and produce deleterious effects.
For instance, the intranasal inactivated influenza vaccine (called NasalFlu, developed by Berna Biotech), which included heat-labile enterotoxin (LT) of the bacterium Escherichia coli as adjuvant, induced Bell’s palsy in several cases. Bell’s palsy is a facial paralysis that results in an inability to control the facial muscles on the affected side. Due to these severe side effects, the vaccine NasalFlu was withdrawn from the market in 2001 (however, afterward alternative safe intranasal flu vaccines were licensed in the USA and India, as discussed in the next part of this series).
Hence, different adjuvants need to be tested for their efficacy to induce a suitable immune response to a given antigen, with minimal or no adverse effects. Therefore, the Food and Drug Administration (FDA) in the USA and other national regulatory agencies in many countries have not licensed adjuvants as separate products but only as components of a given vaccine formulation. Alum is the most extensively used adjuvant, it is a component of licensed vaccines against several pathogens, such as diphtheria, tetanus, pertussis, hepatitis A and hepatitis B.
In the third part of this series, we will see how the vaccine delivery route determines not only the safety of vaccines, but also the location, type and magnitude of the immune response, and consequently the vaccine efficacy.
 News / Judge Business School advisor resigns over Epstein and Andrew links18 February 2026
News / Judge Business School advisor resigns over Epstein and Andrew links18 February 2026 News / Gov grants £36m to Cambridge supercomputer17 February 2026
News / Gov grants £36m to Cambridge supercomputer17 February 2026 News / CUCA members attend Reform rally in London20 February 2026
News / CUCA members attend Reform rally in London20 February 2026 News / Union speakers condemn ‘hateful’ Katie Hopkins speech14 February 2026
News / Union speakers condemn ‘hateful’ Katie Hopkins speech14 February 2026 News / Hundreds of Cambridge academics demand vote on fate of vet course20 February 2026
News / Hundreds of Cambridge academics demand vote on fate of vet course20 February 2026